Reporting Audit Findings
OVERVIEW
BEFORE YOU BEGIN
- Collect the audit report, the response to the audit report, and the management plan that outlines how the findings will be addressed and future occurrences prevented.
STEPS

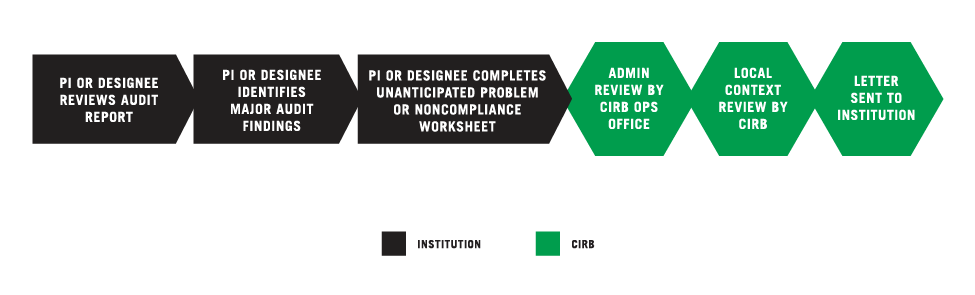
PI OR DESIGNEE REVIEWS AUDIT REPORT
The Principal Investigator (PI) or designee reviews the audit report issued by the institution, a Network, the Office for Human Research Protections (OHRP), or the Food and Drug Administration (FDA) for a study open under the CIRB.

PI OR DESIGNEE IDENTIFIES MAJOR AUDIT FINDINGS
There are two categories of audit findings: major and minor. Any major finding based on the Clinical Trials Monitoring Branch audit guidelines should be submitted to the CIRB as potential serious noncompliance. Any finding that had been documented in a previous audit report should be submitted to the CIRB as potential continuing noncompliance.

PI OR DESIGNEE COMPLETES THE UNANTICIPATED PROBLEM AND/OR NONCOMPLIANCE WORKSHEET
The PI or designee submits any findings (potential serious or continuing noncompliance) using the Unanticipated Problem and/or Noncompliance Worksheet available in IRBManager. For more information, go to the Completing the Unanticipated Problem and/or the Noncompliance Reporting Worksheet.

CIRB OPERATIONS OFFICE CONDUCTS ADMINISTRATIVE REVIEW
The Worksheet is reviewed by the CIRB Operations Office. This review typically takes two weeks. The CIRB Operations Office may send the submitter an email request for additional information. Revisions to the Worksheet are completed in IRBManager.

CIRB LOCAL CONTEXT COMMITTEE CONDUCTS REVIEW
After the administrative review, the Worksheet is sent to the CIRB Local Context Subcommittee for review. This process typically takes five days. The CIRB Operations Office may send the submitter an email request for additional information. Revisions to the Worksheet are completed in IRBManager.

CIRB SENDS DETERMINATION LETTER TO INSTITUTION
After the CIRB Local Context Subcommittee approves the Worksheet, the CIRB Operations Office sends a determination letter to:
- the PI
- the person submitting the Worksheet,
- the Signatory Institution Primary Contact(s), and
- OHRP and FDA if the event is determined to be reportable.