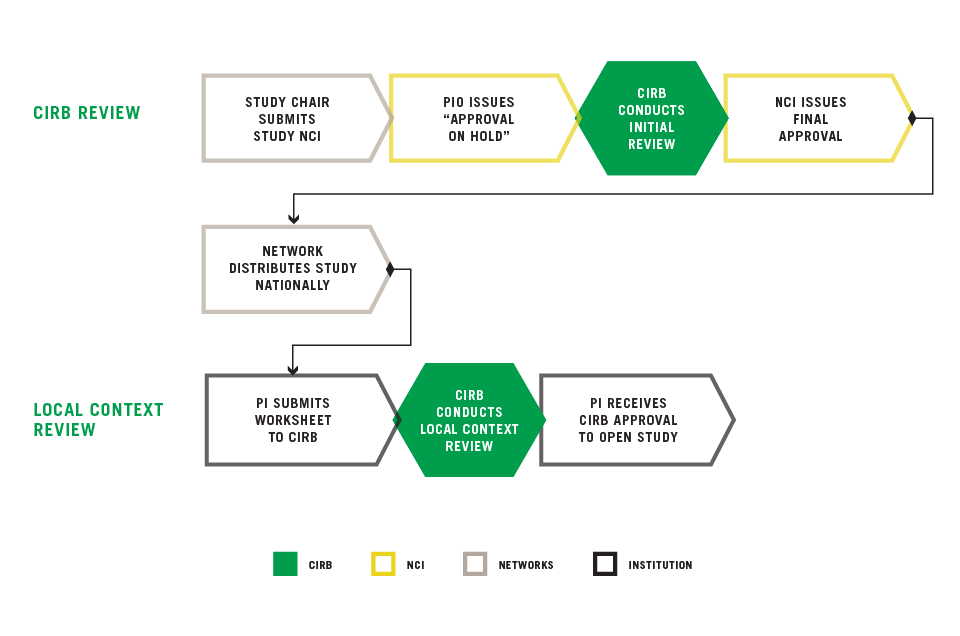
Overview of the Study Review Process
OVERVIEW

STUDY CHAIR SUBMITS STUDY TO NCI
The NCI-funded networks have national Study Chairs. These Study Chairs submit studies to NCI, specifically Cancer Therapy Evaluation Program (CTEP) or Division of Cancer Prevention (DCP), for scientific review.

PIO ISSUES "APPROVAL ON HOLD"
During the scientific review process, CTEP or DCP works directly with the Study Chair to refine the study. Once the Study Chair and CTEP or DCP have completed their review, the Protocol Information Office (PIO) for CTEP or DCP, issues an "Approval on Hold." At this time, the study is received by the CIRB and is pending review and approval by the CIRB.

CIRB CONDUCTS INITIAL REVIEW
Depending on the type of study (i.e., Pediatric, Adult, Phase, etc.), the study is sent to the appropriate board for review. During review of study, the CIRB works directly with the Study Chair to ensure compliance with human subjects protection regulations. Once the CIRB approves the study, CIRB sends an outcome letter to the Study Chair and CTEP or DCP noting that the study has been approved by the CIRB. CIRB notifies PIO upon final CIRB approval and provides the approved consent form and protocol with Approval Letter. For more information, go to Submitting a Study for Initial Review.

NCI ISSUES FINAL APPROVAL
NCI reviews changes required by the CIRB for CIRB approval and issues a final approval to the Study Chair.

NETWORK DISTRIBUTES STUDY NATIONALLY
Once the Study Chair receives final approval, the Study Chair’s NCI-funded Network will distribute the study nationally. The Cancer Trials Support Unit (CTSU) will also have information on most of the studies available on their website under the study’s CIRB documents tab. For more information, go to, Finding Information Q&A.

PI SUBMITS WORKSHEET TO CIRB
Once a study has been distributed, Principal Investigators at Signatory Institutions have the opportunity to open the study. In order to open a study locally, a Principal Investigator (PI) has to be at a Signatory Institution enrolled in the CIRB and the PI must have an approved Principal Investigator Worksheet with the CIRB. To open the study, the PI completes the Study-Specific Worksheet and submits it to the CIRB for review. For more information, go to Opening a Study.

CIRB CONDUCTS LOCAL CONTEXT REVIEW
The Local Context Subcommittee of the CIRB reviews the Study-Specific Worksheet and provides a determination.

PI RECEIVES CIRB APPROVAL TO OPEN STUDY
Once the CIRB Local Context Subcommittee approves the Worksheet, the CIRB Operations Office generates the Approval Letter and sends it to PI. Once the PI has received the Approval Letter, the study may be opened at their institution.